Preparation Of Pancreatic Sections
To investigate the distribution of islet amyloid, three pancreases, designated as pancreases A, B, and C, were obtained from hIAPP transgenic mice. These mice were 15 months old and fed a diet containing 9% fat wt/wt . Each pancreas was fixed in 4% paraformaldehyde in 0.1 mol/l phosphate buffer and embedded in paraffin with the pancreatic head oriented toward the top of the paraffin block. Each pancreas was cut completely, yielding 2,7003,500 sections . We selected 20 sections at a fixed interval throughout each pancreas. The sections were stained with thioflavin-S to visualize islet amyloid.
To determine whether the distribution of -cells in the pancreas differs between hIAPP transgenic and nontransgenic mice, sections were taken from the head, body, and tail of three hIAPP transgenic pancreases and three nontransgenic pancreases , the latter obtained from mice of the same age and genetic background. None of the six mice had been hyperglycemic. The sections were stained by immunofluorescence to visualize the portion of the islet composed of insulin-positive cells.
Type 2 Diabetes Mellitus: A Reciprocal Mutual Interaction With Amyloidosis Or A Presentation Of An Isolated Primary Pancreatic Amyloidosis
Mina T. Kelleni
In two previous editorials, Ive explored some evidence linking type 2 diabetes mellitus to the cytotoxicity of islet amyloid polypeptide aggregates deposited in pancreas and Ive expressed a strong wish and call to focus on the full of potentials amyloid research as well as on drug development of amyloid antagonists. In this short commentary, Im trying to search deeper on the relationship between both diseases and whether T2DM can be a consequence of pancreatic amyloidosis .
Primary amyloidosis was shown to be particularly difficult to diagnose because its signs and symptoms are subtle and because of the absence of specific imaging or laboratory tests, except histopathology . Meanwhile, secondary amyloidosis has been shown to complicate chronic inflammatory autoimmune diseases and chronic diseases such as autoimmune disease and T2DM have also been suggested to be potential factors for AA amyloidosis . Thus, a reciprocal interaction may be also involved several cases of insulin-derived localized amyloidosis have been reported like those mentioned in and a case of primary systemic amyloidosis with the history of T2DM for nine years has also been recently described . Further, Inflammation is known to be closely involved in the pathogenesis of T2DM, and reactive amyloidosis occurs in the presence of chronic inflammation and renal AA amyloidosis was shown to be prevalent in T2DM .
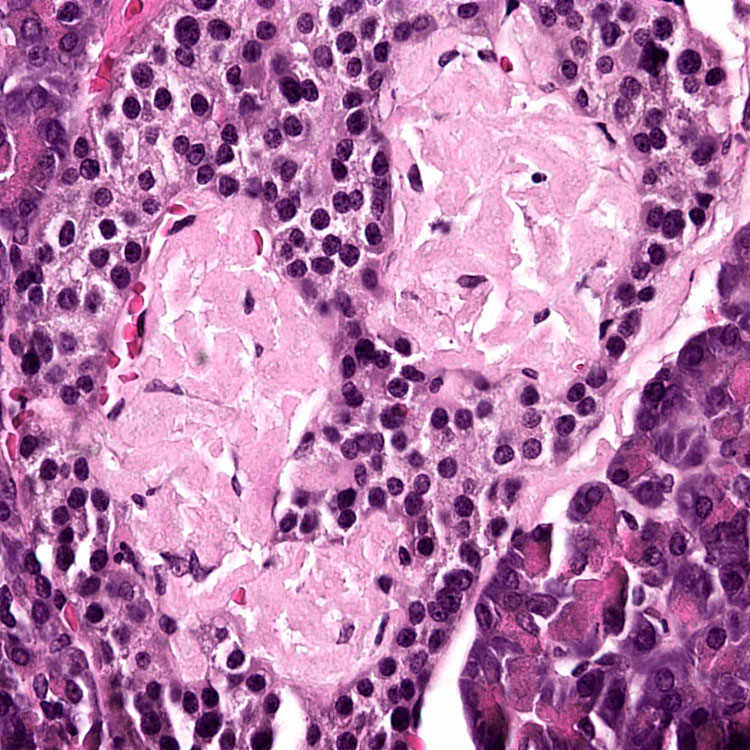
Islet Amyloid Involves Nearly All Islets At An Early Stage
To determine whether a high prevalence of islet amyloid was achieved even when smaller amounts of amyloid were present, we investigated the relation between amyloid prevalence and amyloid severity in the three pancreases described above and in another 12 hIAPP transgenic pancreases that were prepared for the study of islet cell composition. As described in the research design and methods section, the 12 additional pancreases were embedded so as to contain two or three random specimens of a single pancreas in a section. In view of our observation that amyloid deposition was uniform in the pancreas, 20 ± 2 islets on one section were examined in each of the 12 additional pancreases. As shown in , amyloid severity and prevalence from the 15 mice were positively and nonlinearly correlated. After log transformation, the equation between these two parameters was . A high amyloid prevalence was found when total amyloid area was equal to 1.5% of the total islet area, a finding similar to that in the three hIAPP transgenic pancreases. In some sections we did observe variability in the severity of amyloidosis, with some islets containing more amyloid than others. However, in no pancreas did we observe severe islet amyloidosis without a high prevalence of islet involvement.
Recommended Reading: Why Does My Blood Sugar Drop After Eating Protein
Mouse Islet Isolation Homogenization And Culture
Islets from Tg-hIAPP, as well as controls, were isolated using a previously published procedure . The pancreatic tissue was digested with collagenase IV for 15 min at 37°C. The digested material was recovered by centrifugation, washed in HBSS, and filtered through a 70-µm cell strainer. Finally, islets were separated from the remaining exocrine tissue by a Ficoll gradient, as previously described . To prepare islet extracts, isolated islets were weighed and immediately frozen in liquid nitrogen and stored in 80°C. The stock was homogenized in PBS, using a mechanical Eppendorf homogenizer, to prepare 1% homogenate. For islet culture from Tg-hIAPP mice, isolated islets were cultured in RPMI-1640 , supplemented with 10% FBS, glutamine, and antibiotics at 37°C in 5% CO2.
Amyloid Formation Is The Pathological Hallmark Of T2d And Ad
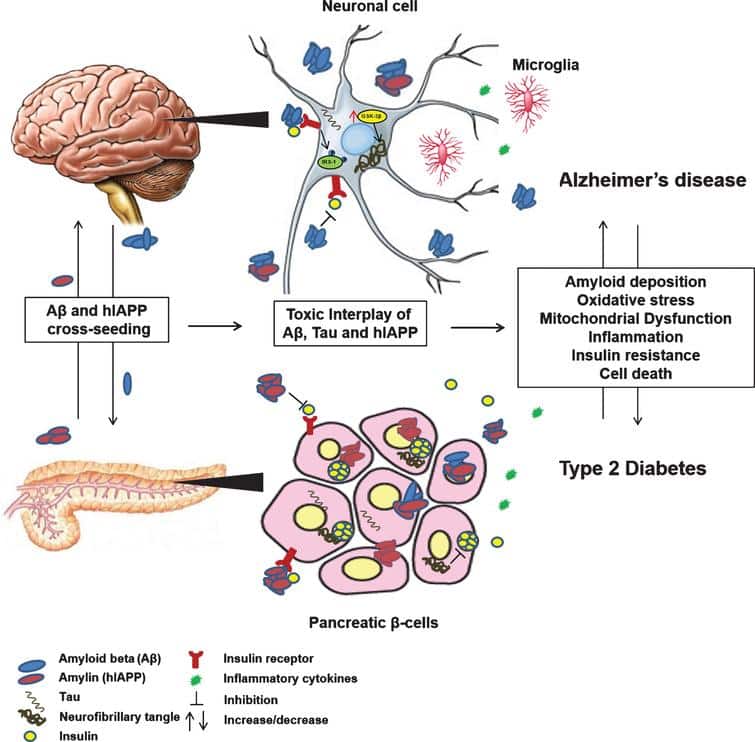
The incidence of both AD and T2D is increasing at an alarming rate at present and has become a major public health concern in many industrialized countries . Many epidemiological studies have shown that diabetic individuals have a significantly higher risk of developing AD . Recently, it has become increasingly recognized that there is an overlap between the pathology of AD and vascular dementia and cerebrovascular dysfunction plays a role not only in vascular dementia but also in AD . Nevertheless, clinical observations suggest that the association is independent of vascular factors , which raises the possibility that diabetic conditions such as insulin resistance and hyperglycemia may affect the fundamental pathogenesis of AD. Many neuronal functions are affected by changes in the insulin signaling pathway therefore diabetes mellitus may have an important role in the progression of AD .
Recommended Reading: Can You Donate A Kidney If You Have Diabetes
Islet Amyloid Polypeptide Synthesis Secretion And Function
In the case of islet amyloid, IAPP is the unique amyloidogenic precursor peptide. IAPP is a normal product of the pancreatic islet -cell and is stored along with insulin in secretory granules . Like insulin, IAPP is derived from a larger propeptide precursor, preproIAPP, that in humans is an 89-amino acid peptide that contains an amino-terminal signal sequence, consistent with this being a secreted peptide . Once the signal peptide is removed, the 67-amino acid propeptide proIAPP is enzymatically cleaved to the mature 37-amino acid peptide IAPP by the prohormone convertases PC1/3 and PC2 , which are also responsible for proteolytic conversion of proinsulin to insulin . Additional posttranslational modifications include formation of a disulfide bridge between cysteine residues at positions 2 and 7 and amidation of the C-terminal tyrosine .
Although a definite physiological function has yet to be clearly ascribed to IAPP, its fundamental role in the formation of islet amyloid in the pancreas of individuals with type 2 diabetes has been clearly defined. In this manner, this islet peptide plays a role in the pathogenesis of the islet -cell dysfunction observed in type 2 diabetes.
Diabetes Mellitus And Amyloid
Clinically, Parkinsons disease is defined as a progressive disorder characterized by resting tremor, rigidity, and bradykinesia however, there may be other manifestations less constant such as postural instability, propulsive gait, dysphagia, autonomic disorders, sebaceous sweating, salivation, and deteriorating superior functions that can lead up to dementia. This disease was described in 1817 by James Parkinson, who described the deficiency of dopamine in the brain of his patients in late 1950 and also described the treatment of this disease with L-dopa in the 1960s. Parkinsons disease is the second most frequent neurodegenerative disorder. It is a motor disease related with the disorder of the basal ganglia specifically via nigrostriatal which is formed by the axons of the dopaminergic neurons of the substance compact nigra which innervate the corpus striatum. This structure is considered the main target of dopaminergic innervation due to the high density of axons it receives and its large size. The main symptoms of Parkinsons disease are caused by the degeneration of dopaminergic neurons via nigrostriatal . A pathological hallmark of Parkinsons disease is the Lewy bodies , eosinophilic inclusions of -synuclein located in the neuronal soma especially in nigra substance . Besides the there can be deposits of the protein tau and of -amyloid .
Figure 3.
Recommended Reading: Can Diabetics Eat Watermelon And Cantaloupe
Research Design And Methods
hIAPP transgenic mice.
Mice expressing the hIAPP gene in their pancreatic -cells were bred at the University of Washington . By breeding male hemizygous hIAPP transgenic mice with female nontransgenic mice , we produced offspring that did or did not express the hIAPP transgene. Genotyping was performed by polymerase chain reaction using primers specific for hIAPP . Because islet amyloid occurs more frequently in male than in female hIAPP transgenic mice , only male mice were studied. Male nontransgenic littermates were used when control mice were necessary. All of the studies were approved by the Animal Care Committee at the VA Puget Sound Health Care System.
Induction Of Iapp Amyloid Deposition And Associated Diabetic Abnormalities By A Prion
A. Mukherjee, D. Morales-Scheihing, and N. Salvadores contributed equally to this paper.
- Abbreviations used:
J Exp Med
Abhisek Mukherjee, Diego Morales-Scheihing, Natalia Salvadores, Ines Moreno-Gonzalez, Cesar Gonzalez, Kathleen Taylor-Presse, Nicolas Mendez, Mohammad Shahnawaz, A. Osama Gaber, Omaima M. Sabek, Daniel W. Fraga, Claudio Soto Induction of IAPP amyloid deposition and associated diabetic abnormalities by a prion-like mechanism. J Exp Med 4 September 2017 214 : 25912610. doi:
Recommended Reading: How To Lower Diabetes Levels
Amyloid Formation As A Common Pathological Feature In Both Diabetes And Alzheimers Disease
2.1. Relations between Diabetes and Alzheimers Disease
2.2. Amyloid Formation and Deposition Involving both Neurodegenerative Changes and Neurovascular Damage
- Misplaced mutations of the APP, presenilin1 , and or presenilin2 genes that may result in increased production of A42 peptides throughout life in the dominant forms of AD or,
2.3. Evidence from the Shared Pathological Traits
Association Of Peripheral Insulin Resistance And Other Markers Of Type 2 Diabetes Mellitus With Brain Amyloid Deposition In Healthy Individuals At Risk Of Dementia
Article type: Short Communication
Authors: Pekkala, Timoa | Hall, Anettea * | Mangialasche, Francescab c | Kemppainen, Ninad e | Mecocci, Patriziaf | Ngandu, Tiiab g | Rinne, Juha O.d e | Soininen, Hilkkaa h | Tuomilehto, Jaakkog i j | Kivipelto, Miiaa b k l | Solomon, Alinaa b
Affiliations: Institute of Clinical Medicine/Neurology, University of Eastern Finland, Kuopio, Finland | Division of Clinical Geriatrics, Center for Alzheimer Research, NVS, Karolinska Institutet, Stockholm, Sweden | Aging Research Center, NVS, Karolinska Institutet and Stockholm University, Stockholm, Sweden | Turku PET Centre, University of Turku, Turku, Finland | Division of Clinical Neurosciences, Turku University Hospital, Turku, Finland | Department of Medicine, Institute of Gerontology and Geriatrics, University of Perugia, Perugia, Italy | Public Health Promotion Unit, Finnish Institute for Health and Welfare, Helsinki, Finland | Neurocenter, Neurology, Kuopio University Hospital, Kuopio, Finland | Department of Public Health, University of Helsinki, Helsinki, Finland | National School of Public Health, Madrid, Spain | Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio, Finland | Ageing Epidemiology Research Unit, School of Public Health, Imperial College London, London, United Kingdom
Keywords: Amyloid-β, apolipoprotein E, plasminogen activator, positron emission tomography, type 2 diabetes
DOI: 10.3233/JAD-200145
Abstract
Read Also: Blood Sugar To A1c Converter
Glucose Tolerance Test And Insulin Secretion
Glucose tolerance tests were performed in mice after 15 h of fasting or 5 h of fasting . The shorter fasting was performed in mice to reduce the stress observed in 20-week-old Avy hIAPP mice when fasted overnight. Mice were intraperitoneally injected with d-glucose , and glucose levels in tail vein blood samples were measured with a blood glucometer just before and 15, 30, 60 and 120 min after glucose injection. Blood samples were taken at just before and 15 min after glucose injection with Microvettes containing EDTA to obtain plasma and measure insulin using an Ultra Sensitive Mouse Insulin ELISA kit .
Localization Of Ttr In Islets Of Langerhans
A cDNA library was constructed fromhuman liver with the aid of a cDNA synthesis kit .A 237-nucleotidelong fragment corresponding to amino acid residues 2106 of TTR was amplifiedby polymerase chain reaction. The achieved fragment was ligated into themultiple cloning site of pGEM4Z .The vector was linearized in front of the SP6 or T7 promoters anddigoxigenin-labelled RNA probes were produced according to the manufacturer. In situ hybridization wasperformed as described .
Forultrastructural studies, pancreatic tissue was available from one patient with type-2diabetes and one normal control, fixed in 2% glutaraldehyde in phosphate bufferand embedded in epon. For double immunolabeling, ultrathin sections onformvar-coated nickel grids, were incubated with the primary antibodies and mouse antiglucagon . TTR was visualized with 5 nmgold particles and glucagon with 10 nm gold particles .
Recommended Reading: Three Risk Factors For Type 2 Diabetes
Approaches To Inhibiting Islet Amyloid Formation
The increasing body of evidence linking aggregation and cytotoxicity of IAPP and islet amyloid formation to a decline in -cell mass and function underscores the importance of developing methods to decrease or prevent islet amyloid formation.
The development of inhibitors of islet amyloid fibril formation is in relatively early stages, although many findings from inhibitor studies targeted at other forms of amyloid, such as A fibril formation in Alzheimers disease, may be applicable to diabetes. Inhibitors based on small molecules such as Congo Red, which bind all amyloid fibrils, have been shown to have effects on IAPP. Although Congo Red does not affect the fibrillogenesis of human IAPP , it has been shown to inhibit the cytotoxic effect of both human IAPP and A . Alternatively, the antibiotic rifampicin or its analogs, through their free radical scavenging ability, have been shown to be effective in inhibiting the cytotoxic effects of human IAPP , providing more evidence for an association between human IAPP cytotoxicity, oxidative stress, and islet amyloid formation. Small molecule analogs of glucosamine, a basic subunit of the GAG chains of heparan sulfate proteoglycans, have also been shown to be effective in blocking GAG chain elongation and thereby inhibiting amyloid formation in vivo in a model of AA amyloidosis .
Role Of Amyloids In Type Ii Diabetes
by Los Alamos National Laboratory
A collaboration between Los Alamos, Yale University, and Worcester Polytechnic Institute published research in the journal Langmuir that sheds light on pathological properties of amyloids identified in type II diabetes. Amyloids are unwanted aggregates of proteins in our bodies. Frequently they form fibers or plaques whose presence is correlated with the pathology for many diseases, including Alzheimer’s, Parkinson’s, and type II diabetes.
The investigators examined interactions of human and rat islet amyloid polypeptides with several formulations of model lipid membranes differing in ratio of neutral to charged molecules, density of their packing and the amount of defects in their structures. Human IAPP is toxic and prone to form amyloids, whereas rat IAPP has reduced toxicity and does not aggregate into fibrils under physiological conditions. However, both peptides are the same length, and their sequences differ by only a few amino acids.
More information:Ann Junghans et al. Influence of the Human and Rat Islet Amyloid Polypeptides on Structure of Phospholipid Bilayers: Neutron Reflectometry and Fluorescence Microscopy Studies, Langmuir . DOI: 10.1021/acs.langmuir.6b00825
Journal information:
Recommended Reading: Free Diabetic Meter And Test Strips
Production Of Synthetic Iapp And Preparation Of Aggregates
hIAPP containing the appropriate posttranslational modification was synthesized using solid-phase N-tert-butyloxycarbonyl chemistry at the W. Keck Facility at Yale University and was purified by reverse-phase HPLC. The final product was lyophilized and characterized by aa analysis and mass spectrometry. To prepare stock solutions free of IAPP-aggregated seeds, lyophilized, synthetic IAPP was dissolved in 2 mM HCl, and the resulting solution was sonicated and centrifuged at 16,000 g to remove existing aggregates. For ex vivo studies and i.p administration, the stock was diluted in PBS to the desired concentration. For in vitro seeding the stock was diluted in 100 mM Tris-HCl, pH 7.4 to reach the desired concentration. To produce IAPP aggregates, solutions of IAPP in PBS were incubated overnight at 37°C with constant shaking at 500 revolutions per min . For the in vitro seeding assay, the stock solution of IAPP in 100 mM Tris-HCL was incubated at 25°C. The degree of aggregation was characterized by ThT fluorescence emission and electron microscopy after negative staining, as previously described .
What Are The Risks Of Islet Transplantation
Risks of islet transplantation include
- bleeding, blood clots, and pain after the procedure
- the chance that the transplanted islets may not work well or may stop working
- side effects of anti-rejection medicines, also called immunosuppressants, which are described below
- development of antibodies against the donor cells that may make it more difficult to find an appropriate organ donor if another transplant is needed in the future
Recommended Reading: What Diet Is Best For Type 1 Diabetes
Pancreatic Iapp Aggregates Induce Endogenous Iapp Deposition In Cultures From Tg Mice And Human Islets
To begin evaluating whether islet amyloid pathology might be transmissible, we performed studies in islets isolated from Tg mice that overexpress hIAPP . It has been shown that mouse IAPP is not amyloidogenic and does not accumulate as aggregates in the pancreas . Male homozygous mice for hIAPP develop T2D-like clinical and pathologic alterations, including hyperglycemia, reduced insulin secretion, IAPP amyloid deposits, and cell loss . IAPP aggregation starts as small, intracellular aggregates and later becomes accumulated as massive, extracellular deposits over time. 12-mo-old Tg mice displayed extensive islet pathology and overt signs of T2D, including massive amyloid deposits, impaired insulin production and severe hyperglycemia . Age-matched, non-Tg mice of the same background, which did not have any detectable IAPP aggregates , were used as controls.