Medtronic Is Expanding A Recall Of Two Of Its Minimed 600 Series Insulin Pumps After Reports Found Several Had Broken Retainer Rings Cracked Resulting In Insulin Doses That Were Too High Or Too Low
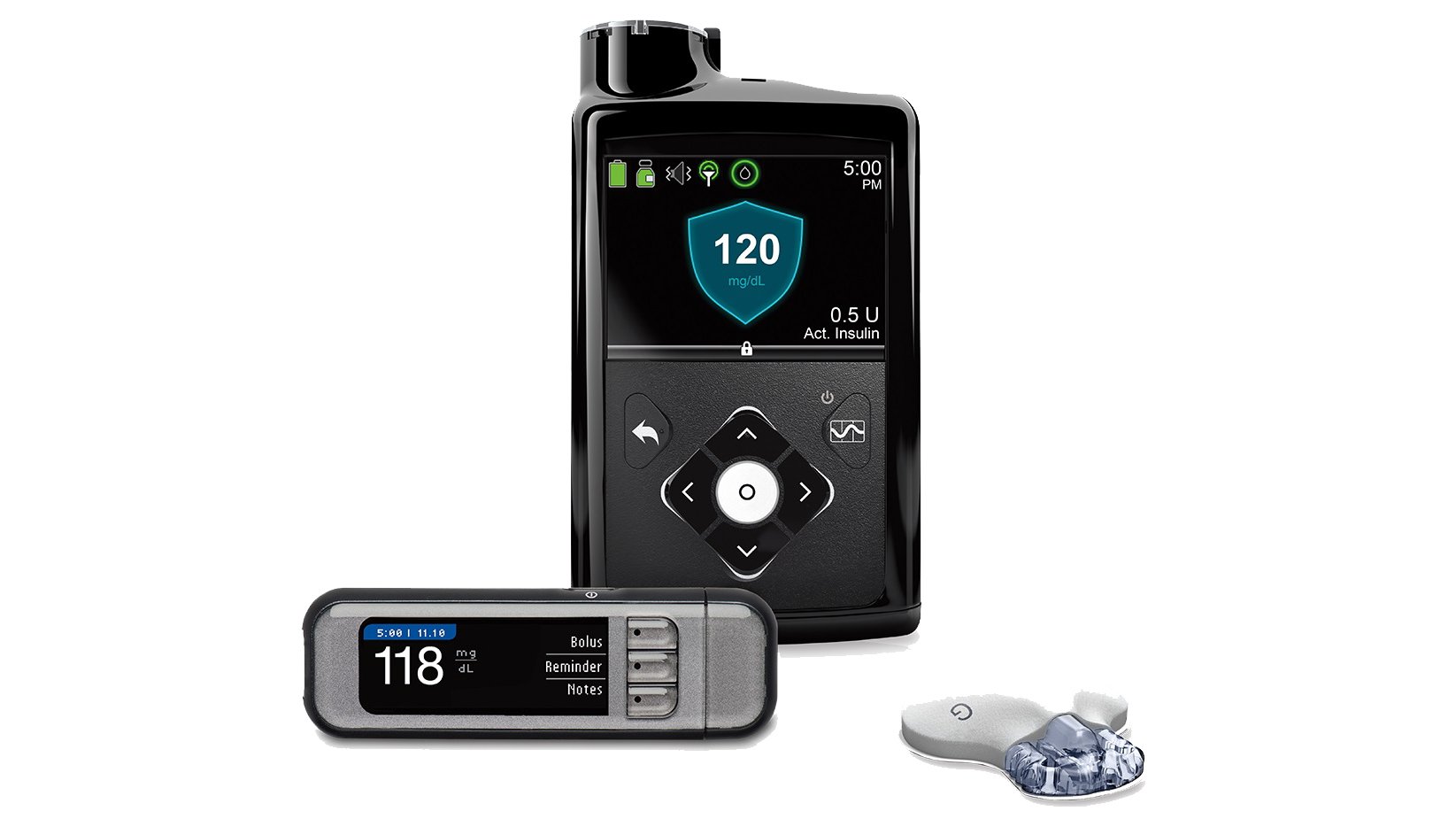
Medtronic is recalling its MiniMed 670G system after reports of broken retainer rings for insulin cartridges leading patients to receive the incorrect dose. Photo credit: Medtronic
Medtronic is expanding a recall of its MiniMed insulin pumps after a broken retainer ring led patients to receive the incorrect dose of insulin.
The recall, first announced in November 2019, was expanded yesterday to include all Minimed 630G and 670G insulin pumps for Medtronic to replace their retainer rings. It includes more than 463,000 of the devices in the U.S.
The clear retainer ring, intended to lock an insulin cartridge into the devices, would sometimes break, resulting in patients receiving either too much or too little insulin. In one complaint filed with the FDAs MAUDE database, a patient said they had the pump replaced for a fourth time due to a crack on the back of the pump, leading to inaccurate readings and severe low blood sugar.
In a February notice, the FDA said it is aware of 2,175 reported injuries and one death. It recently to say that serious injuries and deaths have been reported with the use of the recalled insulin pumps, but not all of those adverse events may have been directly related to the damaged retainer rings.
In a statement posted to its website, Medtronic said it is updating the recall to proactively replace the affected insulin pumps with ones that have black retainer rings designed to better withstand damage from accidental bumps or drops.
Promoted
Medtronic Insulin Pumps Recalled After 1 Killed Thousands Injured From Incorrect Doses
Health care technology company Medtronic recalled more than 300 thousand MiniMed 600 Series insulin pumps Wednesday in a Class I recall after thousands of injuries and one death.
Medtronic recalled all Model 630G pumps from before October 2019 and all Model 670G pumps from before August 2019, according to the FDA. The model numbers for the pumps are MMT-1715 and MMT-1780, respectively. These devices may be used by people who have Type 1 diabetes to administer insulin. The 630G model may be used by people age 16 and older. The 670G model may be used by people 14 years old and older. Besides people with diabetes who use the pump, this may affect health care providers who treat people with diabetes using this pump.
According to the FDA, the affected pumps have a broken or missing retainer ring, which locks the insulin cartridge in the pump’s reservoir compartment. This can cause the under- or over-delivery of insulin if the cartridge is not securely locked in place. Hypoglycemia or hyperglycemia can occur in these instances. Severe hyperglycemia can lead to loss of consciousness, seizure or death.
The FDA identifies a Class I recall as the most serious, where exposure to the product may result in death or adverse health consequences. Medtronic received 26, 421 complaints where the device malfunctioned and is aware of 2,175 injuries in addition to the death.
Medtronic Minimed Insulin Pump Recall Expanded To Include Half A Million Devices
Due to continued problems with dosing, Medtronic is expanding a MiniMed 600 series insulin pump recall to include nearly half a million devices, which could over or under deliver medication. A second, smaller recall impacting more than 30,000 MiniMed and Paradigm pump remote controllers is also being announced, due to cybersecurity risks.
The FDA announced the Medtronic Minimed 600 series recall expansion on October 5, indicating the agency has designated this a class I recall, the most serious recall classification. The designation means the agency believes the pumps may pose an increased risk of injury and death to patients.
The Medtronic MiniMed insulin pumps are small, computerized devices that deliver insulin to diabetic patients throughout the day, via a catheter implanted under the skin. They are wirelessly connected to both the patients blood glucose meter and a monitoring system to track glucose levels, as well as a remote controller that is designed to communicate with the pump and deliver a specific amount of insulin.
Recommended Reading: Which Is Worse Type I Or Ii Diabetes
Medtronic Insulin Pump Recall History
The November 2019, Medtronic MiniMed Insulin Pump recall is a Class I recall, indicating that it has a high risk for potential harm, however it is not the first recall Medtronic has been forced to issue for their insulin pumps.
Other recalls have included:
- 2009 MiniMed Quickset infusion sets for the Paradigm pump recalled due to a manufacturing defect that had resulted in dosing errors
Medtronic Minimed Insulin Pump Recall Lawsuit
Medical device manufacturer, Medtronic has recalled two models of its MiniMed Insulin pumps because of a possibility of life-threatening dosing errors of overdose or underdosage of insulin. People or oved ones of those who were injured by malfunctioning pumps may be considering filing a Medtronic MiniMed Insulin Pump lawsuit.
In November of 2019, the U.S. Food and Drug Administration announced a Class I recall of Medtronic MiniMed Insulin Pumps. The recall may affect 322,005 pumps in the U.S. and was caused by a part of the pump which may be broken or missing and may result in serious injury due to insulin over dosage or under dosage.
The affected MiniMed insulin pump uses a retainer ring to lock the cartridge or reservoir which contains the supply of insulin in place. If the retainer ring is broken or missing, the cartridge may not be properly secured which may lead to over delivery or under delivery of insulin. Such an error may result in hypoglycemia or hyperglycemia and may be life threatening.
Of the 322,005 pumps which may be affected by the recall, there have been 26,421 error reports which included 2,175 injuries and at least one death which have been received by the FDA. People or loved ones of those who were injured by a MiniMed Insulin Pump malfunction may be considering filing a lawsuit against Medtronic to seek compensation.
Read Also: What Is The Correct Reading For Diabetes
What Is The Problem With The Medtronic Minimed Insulin Pumps
In this latest recall, the FDA stated that Medtronics recalled infusion sets had a defective component that is causing the device to over-deliver insulin under certain conditions. The vent membrane inside the set can become blocked during priming and fill-tubing, which can result in insulin over-delivery. The blockage may occur if insulin or other fluids like water are spilled on top of the insulin reservoir during the priming process. Medtronic has advised that it redesigned the vent membrane.
The company stated in a press release that if the infusion sets become blocked, it can lead to potential over-delivery of insulin shortly after an infusion set change, which may cause hypoglycemia.
Medtronic was allegedly unaware of the issue until it started receiving reports from patients describing incidences of hypoglycemia. The company analyzed the device and found that the vent membrane, which has now been discontinued, could cause blockage shortly after an infusion set change.
Newer infusion sets, available since April 2017, include a design update of this component, which the company says reduces the risk of insulin over-delivery after an infusion set change. Yet the company did not offer to recall and replace older units until September 2017.
Medtronic has assured patients it will replace defective infusion sets with new ones containing the updated component at no cost.
Over 300000 Minimed Insulin Pumps Were Recalled
Medtronic has recalled over 300,000 insulin pumps after scores of injuries and one death were reported due to the device malfunctioning, according to the U.S. Food and Drug Administration.
The recalled MiniMed 600 Series models either had a missing or broken retainer ring which helps to lock the insulin cartridge into place in the pump’s reservoir compartment, the FDA said Wednesday. If the cartridge is not locked into place, an inaccurate amount of insulin may be delivered, which can result in hypoglycemia, low level of blood sugar or hyperglycemia, an excess of sugar in someone’s system, the FDA warned. Severe hyperglycemia can result in a loss of consciousness, seizures and death.
Insulin pumps, used to manage diabetes, are computerized devices that deliver small doses of insulin, helping diabetics reach their target blood glucose levels.
Medtronic — MiniMed 600 Series Insulin Pumps
The medical device company initiated the recall in November after discovering it’s MiniMed 600 Series insulin pumps were delivering inaccurate does of insulin, according to the FDA.
CVS OFFERS OPTION FOR DIABETES DRUGS WITH NO OUT-OF-POCKET COST
Approximately 322,005 devices have been affected.
The recalled products include the following:
- Model 630G.: This model was distributed between September 2016 to October 2019.
- Model 670G : This model was distributed between June 2017 to August 2019.
The pump model number can be found directly on the bottom or on the back of the device.
You May Like: Insulin Plant For Type 1 Diabetes
Over 31000 Devices To Be Recalled
According to the FDA, more than 31,000 devices in the United States have been recalled. Medtronic and the FDA also noted that users whose devices were under warranty were informed about the issues back in August 2018. Medtronic further explained that the recall would be expanded to the optional remote controllers compatible with the affected insulin pumps.
SEE: Medicine pumps, Pacemaker threat as Drs simulate hacked overdose
The device users have been sent updated instructions and informed about the issue with impacted controllers. The company urges the users of these pumps to stop using the controllers and return them to Medtronic. Medtronic also explained that the risks associated with MiniMed remote controller outweigh the benefits of its continued use.
Did you enjoy reading this article? Like our page on and follow us on .
Medtronic Says To Stop Using These Old Minimed Remotes
Shortly after issuing the first recall update notice, FDA sent out another notice Tuesday explaining that Medtronic updated another recall related to the MiniMed technology. This time, the company expanded a 2018 recall of remote controllers used with either the MiniMed 508 insulin pump or the MiniMed Paradigm family of insulin pumps due to potential cybersecurity risks. Medtronic has instructed people who may still be using the remote controllers to stop using it, and to return it.
The remote controllers impacted by the cybersecurity issue are older models that use previous-generation technology, and FDA said that as of July 2018, Medtronic is no longer making or distributing these remote controllers.
FDA said it is not currently aware of any reports of patient harm related to these potential cybersecurity risks. Still, it is scary to think about what could happen if a hacker did take advantage of this cybersecurity vulnerability, and it is a good reminder of why cybersecurity is so critical in medtech . When the recall was originally issued, FDA said a hacker could potentially connect wirelessly to a nearby device and change the pump’s settings. If that were to happen , it could result in the over- or under-delivery of insulin to a patient using one of these lifesaving insulin pumps.
Read Also: How To Tell If Your Child Has Type 1 Diabetes
Discovering A Design Flaw
In November 2019, Medtronic issued a patient safety alert letter warning that if a piece of the pump hardware, the retainer ring, breaks or becomes loose, the insulin reservoir may shift and prevent a secure lock within the pump. Improper locking can lead to improper insulin delivery that could result in hypoglycemia or hyperglycemia. Though not mentioned in the letter, additional symptoms from improper insulin dosing can include hunger, shakiness, feelings of anxiety or weakness, dizziness, confusion, or difficulty speaking.
The company mentioned that dropping or bumping the device on a hard surface could break a retainer ring on the cartridge. Users were urged to check the rings on their devices and discontinue use if they were broken. If the reservoir was properly locked in place by the retainer ring, Medtronic said patients could continue to use their pump.
The Dangers Of Hypoglycemia
Hypoglycemia is a constant worry for diabetics. Also known as low blood sugar, hypoglycemia occurs when the level of glucose in your blood drops below a normal level. This is typically a level of 70 milligrams per deciliter or less. However, many patients normal numbers may vary depending on their particular condition.
Symptoms of low blood sugar can develop quickly and without warning. In some cases, patients may not experience any symptoms. This is a very dangerous situation because the lack of symptoms does not mean that damage to the body isnt occurring. When blood sugars become too low, patients need to be treated immediately as this is a medical emergency due to the dangers it presents.
Don’t Miss: Patho Of Type 2 Diabetes
Not The First Medtronic Minimed Insulin Pump Recall
This isnt the first time Medtronic has had to recall defective insulin pumps and related components. In , it implemented a Class I recall for 11 million MiniMed Paradigm Infusion sets. The issue was very similar to that addressed with the latest MiniMed recall if fluids came into contact with the inside of the infusion set, it could cause it to malfunction and over- or under-deliver the amount of insulin.
In 2013, the FDA sent a warning letter to the company notifying it that its Northridge, California facility, which manufactured the Paradigm Insulin Infusion Pumps, was not in conformity with current good manufacturing practices. Specifically, the company failed to identify the actions needed to correct and prevent recurrence of the Paradigm Insulin Infusion Pumps device failure.
In 2014, Medtronic recalled over 550,000 MiniMed Paradigm Insulin Infusion Pumps because accidental button pressing errors could occur. The company had received reports that patients had accidentally programmed the pump to deliver the maximum amount of insulin, again, risking hypoglycemia.
Medtronic Lawsuit Settlement Payout
While many lawsuits have been launched against Medtronic by those who have suffered injuries due to defective insulin pumps, the MiniMed lawsuits are still at an early stage, and not all evidence has come to light. The key questions include what Medtronic knew about the faulty pumps, whether it was negligent in its manufacturing process, and whether it willfully tried to cover up the MiniMed insulin pump dangers in order to make a profit. The Medtronic insulin pump lawsuits range from those seeking product liability compensation for being sold a defective device to those who allege defective insulin pumps caused them serious injury. The level of Medtronic payout settlement someone will receive would likely depend on individual circumstances, i.e., level of injury, loss of earnings, medical bills , and so on.
You May Like: Diabetic Fasting Blood Sugar Goal
Minimed Insulin Pump Lawsuits Detail Diabetic Coma And Death
Her complaint said she refilled her pump before going to bed in February 2017. The device malfunctioned and delivered the full amount into her body all at once, causing her blood sugar level to drop. She ate honey to raise it, but lost consciousness for most of the night.
When she came to at 5:30 the next morning, she was disoriented, confused and experiencing impaired vision, according to her complaint. Pain, bruising, cuts and other injuries led her to believe she had suffered hypoglycemic seizures while she was unconscious.
Medtronic Lawsuit Settlement News & Update
A huge number of defective MiniMed insulin pump lawsuits have been launched over the last two years, and there is a call from lawyers for others affected by the pumps to come forward to pursue compensation. As of 2022, the lawsuits are still at an early stage. As for MiniMed payout settlements, much will depend on the evidence of Medtronics actions during the last six years, including when it knew about the faulty MiniMed devices and whether it tried to cover it up. Medtronic settlement amounts will also depend on the level of injury caused in those who have used the companys defective insulin pumps, as well as additional factors such as the loss of earnings, medical bills, and so on.
Perhaps more damning was the FDAs criticisms of how Medtronic acted when it first became aware of the faulty MiniMed insulin pumps. One expert told the Star Tribune that Medtronics violations were serious and severe, pointing to the fact that Medtronic has received 100s of complaints about MiniMed pumps as far back as 2016, but it decided to scrap plans to alert customers. It seems mindboggling that more than three years elapsed between that time and November 2019 when Medtronic finally issued a warning and product recall. To stress the point, these faulty insulin pumps can cause serious injury and death, which Medtronic seemed to ignore for over three years! This fact and others will likely form key evidence for Medtronics lawsuits and potential compensation payout.
You May Like: Type 2 Diabetes Can It Be Reversed
Medtronic Recalls Some Insulin Pumps As Fda Warns They Can Be Hacked
- “MiniMed 508” Medtronic insulin pumps have cybersecurity problems that can’t be updated or patched, and the company is recalling them as a result, the Food and Drug Administration said Thursday.
- It’s a rare example of a medical device recall over a cybersecurity issue, although security professionals and the FDA have raised numerous concerns over the vulnerability of these devices for years.
- The insulin pumps subject to the recall connect wirelessly to other insulin equpiment, including glucose meters, a monitoring system and controls that pump insulin.
Medtronic is recalling some models of insulin pumps that are open to hacks, and the Food and Drug Administration warned consumers on Thursday that they cannot be patched to fix the holes.
It’s a rare example of a medical device recall over a cybersecurity issue, although security professionals and the FDA have raised numerous concerns over the vulnerability of these devices for years.
The insulin pumps subject to the recall connect wirelessly to other insulin equipment, including glucose meters, a monitoring system and controls that pump insulin.
The MiniMed 508 pumps can’t be updated to address security flaws in the device’s firmware, according to the notice. The company is offering alternatives with “enhanced built-in security capabilities.”
The spokesperson also said the company and FDA are not aware of any confirmed reports of a cyberattack on the pumps.
Medtronic’s stock was steady Thursday.