Why Switch Between Basal Insulins
There are various reasons for switching between basal insulins, both medical and practical . Recently, it has been shown that patients at risk of hypoglycemia had improved time in therapeutic range with insulin degludec 100 units/mL compared to glargine 100 units/mL . Furthermore, patients whose weight-based insulin doses are sufficiently large that the volume of injection causes substantial discomfort may want to switch to a basal insulin of smaller unit/volume .
Reasons for switching between basal insulins.
The Unique Mechanism Of Protraction And Pk/pd Profile Of Degludec
Structurally, degludec has similarities to insulin detemir in that it is acylated with a side chain attached to the B29 amino acid. However, the side chain of degludec differs from that of insulin detemir in being a hydrophilic fatty diacid linked via a spacer . This molecular structure allows degludec to bind reversibly to albumin , but it also results in the self-association state of degludec changing from dihexamer to multihexamer chains after injection. These chains subsequently release monomers at a slow and steady rate, which is the primary mechanism of protraction . Another spin-off benefit of the molecular structure of degludec is that it can be co-formulated with other insulins and GLP-1RAs. Co-formulations of previously available insulins was impossible as a result of chemical incompatibilities or the formation of hybrid insulin hexamers with unpredictable absorption kinetics . Consequently, degludec has been developed as two co-formulation products, one with the rapid-acting insulin aspart , and one with the GLP-1RA, liraglutide .
Is Tresiba Or Lantus More Effective
A meta-analysis of 15 clinical studies published in 2019 looked at data from more than 16,000 participants combined. Tresiba produced a larger decrease in blood sugar levels, but overall, the effects of Tresiba and Lantus on hemoglobin A1C were similar. Tresiba did have a statistically significant less chance of causing hypoglycemia. Researchers concluded that while overall glycemic control was similar, Tresiba may be preferred due to the decreased chance of hypoglycemia.
A meta-analysis published in 2018 had similar findings. Tresiba was significantly less likely to cause hypoglycemia events. For this reason, Tresiba may be preferred over Lantus.
This article is not intended to provide medical advice. Your healthcare provider will decide which types of insulin options are best for you.
You May Like: Symptoms Of High Blood Sugar Levels In Type 2 Diabetes
Which Patients Are Suitable For Basal Insulin
The efficacy, safety, and convenience of basal insulins make them suitable for most patients with T2D who require treatment intensification in combination with most other classes of glucose-lowering agents. Despite this, there may be an increased risk of hypoglycemia when combining basal insulin with sulfonylurea both agents are associated with hypoglycemic events, albeit that newer sulfonylureas may carry a lower risk . Thus, we generally recommend gradually tapering sulfonylurea once insulin is initiated. Discontinuation of concomitant thiazolidinedione is also suggested in patients at risk of heart failure .
Given its greater affordability, patients who are well controlled on NPH may be able to continue on this therapy, and reductions in severe hypoglycemia with newer basal insulin analogs seen in clinical trials may be modest compared with NPH in clinical practice . Although GLP-1RAs are usually recommended before insulin , for cost reasons, insulin is often the first injectable used. Common scenarios associated with patient suitability for insulin are shown in Figure 1.
People with type 2 diabetes who are candidates for basal insulin. HbA1c: glycated hemoglobin T2D: type 2 diabetes.
What Is Insulin Degludec
Insulin degludec is a long-acting basal human insulin analog that is used to improve glycemic control in people with diabetes.
Insulin is a hormone produced by your body that helps you to lower your blood glucose levels.
People with type 1 diabetes and some people with type 2 diabetes need to administer man-made forms of insulin. This is because they don’t produce enough insulin themselves or their body doesn’t respond well enough to the insulin they produce.
Basal insulin analogs like insulin degludec, work to keep your blood glucose levels stable during times when you’re fasting, such as at night when you’re asleep. Insulin degludec provides a ‘background’, slow-acting supply of insulin.
Insulin degludec was approved by the US Food and Drug Administration in 2015. The Tresiba brand of insulin degludec is the only version of this medication currently available.
Recommended Reading: Can Type 2 Diabetics Get Dka
Dosage Guide For Converting Tresiba To Lantus :
When switching from a long-acting basal insulin , some diabetes experts do not recommend adjusting the dose. This is especially true when switching from glargin to detemir or detemir to glargin.
According to the ADA , the Endocrine Society, and the JDRF, the following recommendations were recommended when switching from Tresiba to Lantus, Lantus to Tresiba, or switching from one basal insulin to another basal insulin:
Insulin Degludec Vs Glargine
October 4, 2021, 10:20 pm198 Views
Insulin Degludec Vs Glargine is a comparison of two long-acting insulins. They are the longer-acting insulins used commonly as basal insulins to provide insulin cover throughout the day. All insulins have the same mechanism of action.
Insulin is an anabolic hormone that regulates the metabolic pathways of carbohydrates, fats, and proteins.
Apart from carbohydrates, insulin also enhances the uptake of proteins and potassium into the cells. This post on Insulin Degludec vs Glargine is a review of the two insulins.
Which insulin is better, cheap, safe, effective in controlling blood glucose, and has a better cardiac safety profile?
Don’t Miss: Which Rice Is Good For Diabetic Patients
Sensitivity Analyses Of The Primary Endpoint
Four sensitivity analyses were conducted to determine whether deviations in diary entry and missing data affected the primary endpoint results. Sensitivity analysis 1 was based on the 26- to 30-day diary period, where the dates of hypoglycemic events recorded on the hypoglycemia page fell within the diary period. Sensitivity analyses 2 and 3 were based on all diaries regardless of duration, where the dates of hypoglycemic events recorded on the hypoglycemia page matched the events in the diary exactly or fell within the diary period, respectively. Sensitivity analysis 4 was similar to the primary analysis but was based on patients who completed 12-months of follow-up and had a minimum of 23 diary days for each of the five diary periods.
What Are The Main Differences Between Tresiba And Lantus
Tresiba is a prescription-only injectable basal insulin used in the treatment of both Type 1 and Type 2 diabetes mellitus and is manufactured by Novo Nordisk, Inc. Tresiba facilitates the reuptake of glucose into muscle and adipose tissues. Insulin also plays a role in regulating fat and protein metabolism. Biosynthetic insulins act as a replacement therapy to help diabetic patients restore their fat, protein, and carbohydrate utilization.
Tresibas half-life is 25 hours and has no obvious peak. Tresibas long duration of action provides fairly consistent blood sugar control throughout the day while only being dosed once daily. There is no generic version of Tresiba available.
Lantus is also a prescription-only injectable basal insulin used in the treatment of both Type 1 and Type 2 diabetes mellitus. Lantus is manufactured by Sanofi. Lantus and Tresiba work in a similar manner to promote the proper utilization of fat, protein, and carbohydrates in diabetic patients. The half-life of Lantus is about 12 hours and typically dosed once per day. Lantus is delivered subcutaneously and is available as an injectable solution in a 10 ml vial in a concentration of 100 units/ml. It is also available in a Lantus Solostar pen delivery device in the same concentration.
| Main differences between Tresiba and Lantus |
|---|
| Tresiba |
| Yes |
Don’t Miss: How Much Sugar Diabetes Type 2
Coverage And Cost Comparison Of Tresiba Vs Lantus
Tresiba is a prescription insulin that is typically covered by commercial insurances and Medicare drug plans. With some plans, there may be formulary restrictions, and you may want to check your coverage with your plan or pharmacy. The average cost of one 3 ml pen of Tresiba Flextouch 100 unit/ml is about $400 without insurance. You could pay a discounted price of about $350 with a coupon from SingleCare.
Lantus is a prescription insulin that is typically covered by commercial insurances as well as many Medicare drug plans. Formulary restrictions may also affect Lantus coverage. The average cost of one 3 ml pen is about $100, but with a coupon from SingleCare, you could pay about $70.
It is important to note that one pen of each type of insulin will not last the same amount of time for each patient. This is dependent upon the insulin dose prescribed for each patient.
| Tresiba |
| $70+ |
The Switch1 Trial: Proving That Lantus/basaglar Has A Higher Hypocglycaemia Rate Than Tresiba
Lets start with the SWITCH 1 trial. What is it? It was a Phase 3a trial to compare the hypoglycaemia benefit of the two insulins. It was a double-blind, crossover trial, which essentially means that when participating, you used both insulins and didnt know which one you had at any point in the trial. The results were published much earlier this year, but the interesting point in the study seems not to have been picked up on by many in the diabetes world. If you want the full details of the trial design, please take a look here . Its important to note that for this trial, there were 501 initial participants and that 388 of them had factors that increased their risk of hypoglycaemia . There was a 16-week titration period followed by a 16-week maintenance period, where the basal level was considered to be correct. At the end of each 32 week period, the treatments were swapped so that all participants used both treatments. If you want to see the results, please click here. I think youll be unpleasantly surprised. I was. What was being compared? The hypoglycaemic effects of Degludec versus those of Glargine were being compared, and one might argue that the results are disturbing. Why? Because there is a clear difference in what was determined much to the detriment of Glargine. And one that should make decisions on choice of insulin very clear. Overall Hypoglycaemic Risk What you can see here is that during the mainContinue reading > >
You May Like: Diabetes High And Low Symptoms
The Clinical Profile Of Degludec: What Have We Learned From More Recent Studies
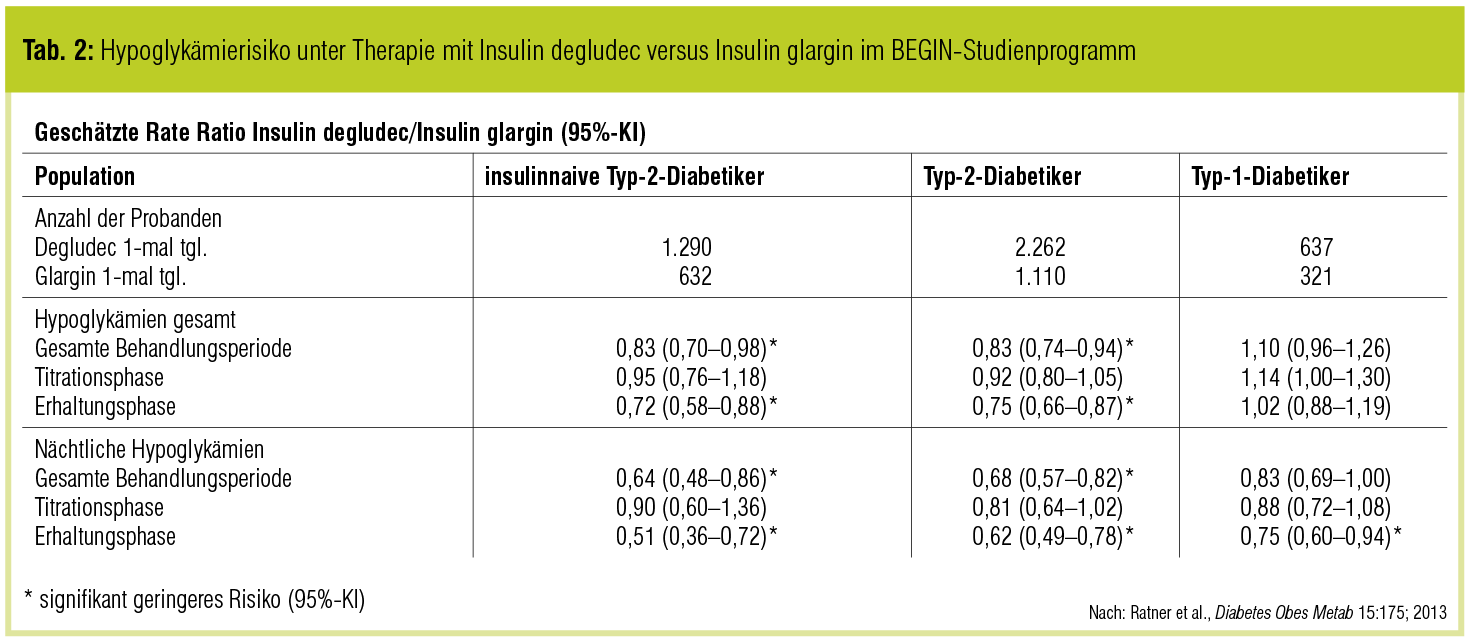
To further assess the relative risk of hypoglycemia with degludec, the US Food and Drug Administration recommended two blinded crossover trialsthe SWITCH studies, which included at-risk patients who would have been excluded from traditional insulin trials . In these TTT studies, patients were initially randomized to either degludec or glargine U100 treatment arms, and then at 32 weeks switched to the opposite treatment arm. During the full treatment period, T1D patients using degludec had a significantly lower rate of overall symptomatic hypoglycemia compared with those using glargine U100 and a significantly lower rate of nocturnal symptomatic hypoglycemia . In the T2D SWITCH study, the respective estimated rate ratios were 0.77 and 0.75 , again favoring degludec. The rate of severe hypoglycemia was significantly lower for degludec over the full treatment period for patients with T1D and T2D .
Switching From One Basal Insulin Analog To Another One
![[Full text] Concentrated insulins: the new basal insulins](https://www.diabetesprohelp.com/wp-content/uploads/full-text-concentrated-insulins-the-new-basal-insulins-tcrm.png)
Basal Insulin from
- Lantus or Basaglar
- Toujeo
Reduce the dose of newly prescribed insulin by 20%.
For Example:
A person on 40 mg insulin Levemir may be switched to Injection Lantus or Toujeo in a dose of 32 units .
According to UpToDate, When switching from basal insulin to Insulin Tresiba, no dosage adjustment is needed.
- Injection Levemir ,
- Injection Lantus or Basaglar ,
- Injection Toujeo ,
Don’t Miss: Why Do You Pee So Much When You Have Diabetes
Cardiac Safety Of Insulin Degludec Vs Glargine:
This area is yet to be explored. However, some insights from a few studies are mentioned here. The DEVOTE 7 Trial concluded that both insulins Deglduec and glargine were associated with increased cardiovascular deaths in patients aged 65 years or more, however, participants in the insulin degludec had fewer episodes of hypoglycemia .
In a Danish cohort study, the risk of death was two-fold reduced in patients who were on insulin Degludec compared to insulin glargine , although, there were no significant differences in the incidence of major cardiovascular events .
Cardiac Safety of Both Insulin Degludec and Glargine are Comparable
What Is The Cost Of Tresiba And Lantus
Whether you have health insurance or not, cost may be a factor when youre considering these drugs. To see cost estimates for Tresiba and Lantus based on where you live, visit GoodRx.com. But keep in mind that what youll pay for either drug will depend on your treatment plan, health insurance, and the pharmacy you use.
Tresiba and Lantus are both brand-name medications. There isnt currently a generic version of either drug.
Lantus is a biologic medication, which means its made from living cells. Although there isnt a generic form of Lantus, theres a follow-on insulin glargine drug available called Basaglar. Follow-on insulins are biologic drugs that are very similar to the original brand-name drug. Basaglar is made with the same type of insulin as Lantus.
However, follow-ons arent considered true generic drugs. This is because the way biologic drugs are made is very complex, and true copies of the original drug cant be made.
Youll usually pay more for the original brand-name drugs than for generics or follow-on drugs.
If youre interested in using Basaglar instead of Lantus, talk with your doctor.
Tresiba and Lantus are both prescribed to help people with type 1 or type 2 diabetes manage their blood sugar. The American Diabetes Associations guidelines recommend both drugs as treatment options for certain people with either type of diabetes.
Read Also: How Do You Treat Diabetic Retinopathy
Common Side Effects Of Tresiba Vs Lantus
Tresiba and Lantus both have the ability to cause hypoglycemia, or low blood sugar. This tendency tends to vary based on factors such as whether the patient is a Type 1 or Type 2 diabetic, what other insulin or diabetic therapies the patient may be using, and diet. When using Tresiba or Lantus with short-acting or rapid-acting insulins, this risk increases.
Patients must have the ability to monitor their blood glucose levels with either a traditional meter or a continuous glucose monitoring system . Patients should also be taught the signs and symptoms of severe hypoglycemia, as it can be life-threatening. These include shakiness, lightheadedness, mental confusion, nausea, blurred vision, and headache. Hypoglycemia can be reversed with the ingestion of glucose or administration of injectable Glucagon.
Injection site reactions may be bothersome to the patient. These can include redness, itching, or bruising. Rotating injection sites can help reduce or alleviate these symptoms.
This list is not intended to be a comprehensive list of potential side effects. Please consult your healthcare provider for a complete list.
| Tresiba |
What Are The Ingredients In Tresiba And Lantus
Tresibas active drug is insulin degludec. Lantuss active drug is insulin glargine.
Both active drugs are long-acting insulins. This means they work over time to keep your blood sugar levels steady throughout the day, in between meals, and during the night. Tresiba works for up to 42 hours, and Lantus works for up to 24 hours.
Tresiba and Lantus are both prescribed to help people with diabetes manage their blood sugar levels. The lists below give details on the uses of each drug.
- Tresiba and Lantus are both used to:
- manage blood sugar levels in adults with type 1 or type 2 diabetes
Note: Tresiba and Lantus arent approved to treat diabetes ketoacidosis . This is a serious, life threatening complication of diabetes. If you have questions about DKA, talk with your doctor.
Tresiba and Lantus both come as liquid solutions that are available in the following forms:
- Vials. With the vials, you use a new syringe and needle for each dose.
- Prefilled pens. The solution is already inside these pens. You use a new needle for each dose. You discard the pen once the doses are used up . The Tresiba prefilled pens are called FlexTouch, and the Lantus pens are called SoloStar.
Both drugs are given by subcutaneous injection . And theyre both typically used once per day.
Recommended Reading: What Is Worse Diabetes 1 Or Diabetes 2
Who May Benefit From Use Of A Longer
Considering these longer-acting basal insulins are more costly than other intermediate- and long-acting basal insulin options, who may benefit from the potential advantages that these longer-acting basal insulins have to offer?
- Patients in whom their current basal insulin does not last a full 24 hours. While many patients do well on once-daily U100 insulin glargine or insulin detemir, some patients experience end-of-dose wearing off, where their blood glucose tends to rise at the end of the dosing interval. Transitioning such a patient to a longer-acting basal insulin product can help them achieve better fasting glucose control with a single daily injection.
- Patients with hectic/erratic schedules. For patients with inconsistent work/life schedules that makes dosing their basal insulin at the same time each day difficult or impossible, switching to a longer-acting product may allow for flexible insulin dosing.
- Patients who require large daily doses of basal insulin. For patients with large basal insulin requirements, the U200 insulin degludec product allows for the administration of up to 160 units in a single injection.
- Patients experiencing nocturnal hypoglycemia with their current basal insulin. Longer-acting basal insulin products have been shown to cause less nocturnal hypoglycemia when compared with U100 insulin glargine .